REGULATORY COMPLIANCE
PHARMACEUTICAL, NUTRITIONALS, COSMETICS AND MEDICAL DEVICE
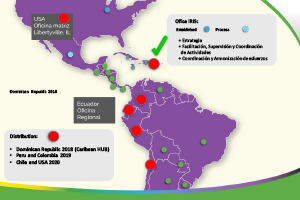
IRIS GLOBAL works with clients on a variety of commercial management models. With an innovative "Globlocal®" strategy, IRIS GLOBAL acts as its main regulatory partner within a country or across the region.
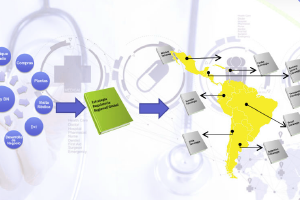
The IRIS GLOBAL team of specialists in Ecuador, the United States and other countries in Latin America offers a complete set of professional skills such as:
• Registration of products before the health authorities LATAM (Pharmaceutical, Nutritional, Cosmetics, Devices).
• Assessment of current capabilities and their compliance: establish the performance of regulations in one or more countries, and detection of strengths, opportunities, weaknesses and threats in the current regulatory approach.
• Develop product submission strategy in Latin America: IRIS GLOBAL builds country-by-country strategies and integrate them into a cohesive and coherent strategy for the region.

• Development of product submission strategies: IRIS GLOBAL works with commercial and development teams to ensure that the consideration of regulatory issues is taken into account in product development proactively.
• Manage product life cycle: IRIS GLOBAL prepares filings and updates based on product life-cycle changes to assure ongoing compliance and market access.
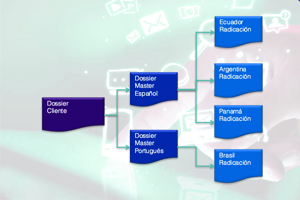
• Prepare and manage dossiers through approval: IRIS GLOBAL prepares master dossiers, translate them into the local language, and submit to health authorities for approval.
• IRIS GLOBAL helps you to be ahead of the curve by learning about regulatory trends and changes before they happen.
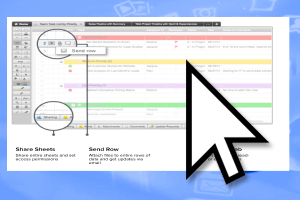
During the development of the project, the IRIS Team implements tools and systems in the cloud, which guarantee a constant and safe flow of information and help to visualize all the advances of the project minute by minute.
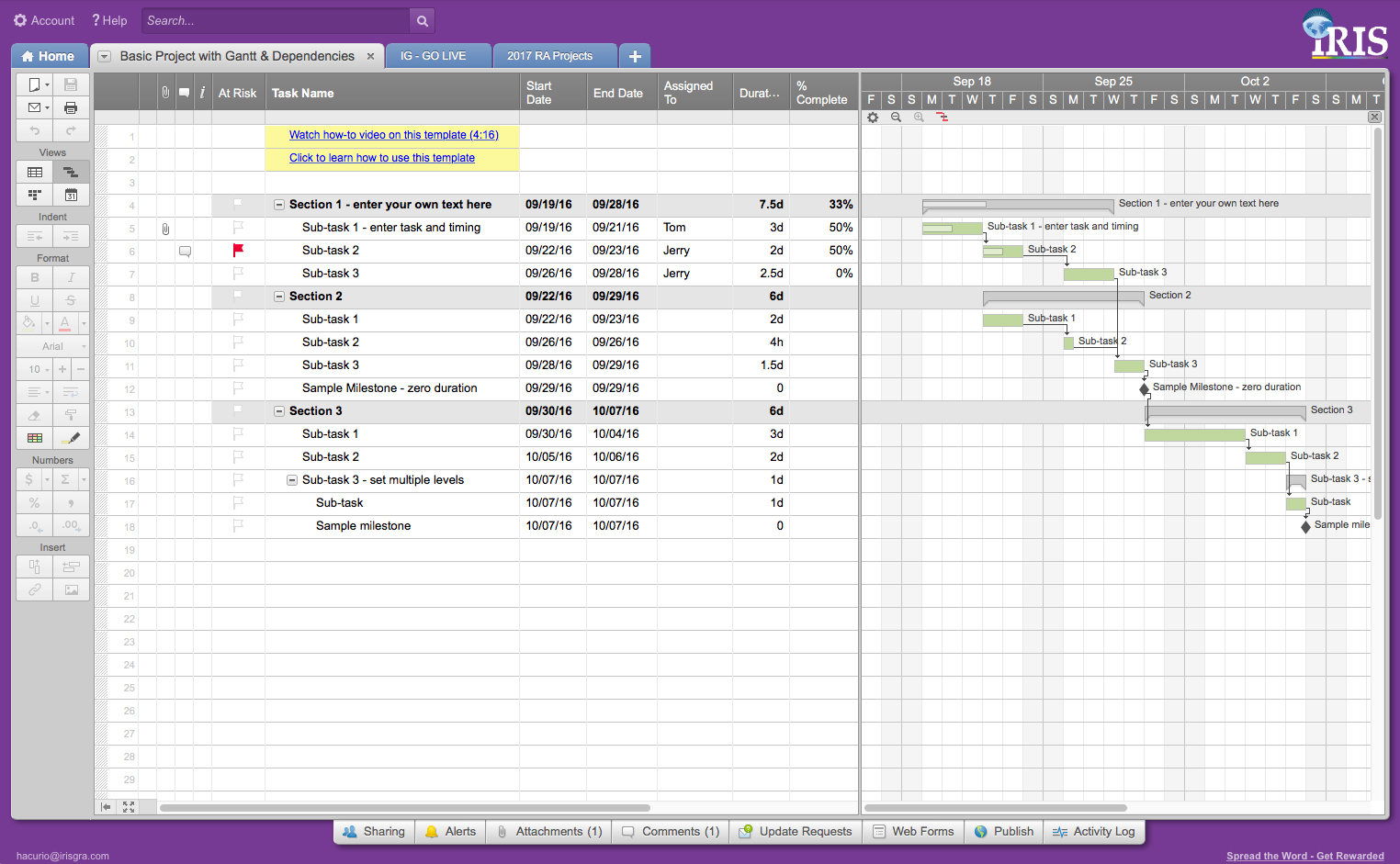
With IRIS GLOBAL, make the regulatory complexity of Latin America, a competitive advantage